β-sitosterol reduce cholesterol levels in high cholesterol diet fed zebrafish
Volume 2, Issue 1, February 2015, Pages 53–65
Mst Kaniz Fatema1![]()
![]() , Zhen-yu Chen2, Ge Wei3
, Zhen-yu Chen2, Ge Wei3
1Research Associate, Department of Biology, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong
2Professor, Department of Biochemistry, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong
3Professor, Department of Biology, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong
| ABSTRACT | Get Full Text PDF |
Cholesterol-fed adult zebrafish (Danio rerio) is a novel model organism for study of lipid signaling and metabolism that can impact on a variety of heart diseases in humans. It also acts as a model system for drug discovery that could affect development of human atherosclerosis. Thus, we investigated the effects of b-sitosterol ingestion on lipid accumulation caused after high cholesterol feeding adult zebrafish. b-sitosterol is a plant sterol that closely resembles cholesterol in terms of chemical structure and is therefore, used sometimes in management of hypercholesterolemia in humans. Forty five days old zebrafish were fed with experimental diets consisting of 1% cholesterol only, 1% cholesterol + 1% b-sitosterol, 4% cholesterol only and 4% cholesterol + 4% b-sitosterol for 5 weeks. Total cholesterol content was significantly lower in groups that were fed diets containing 1% and 4% b-sitosterol than in those that were fed high cholesterol diet only. Both 1% and 4% β-sitosterol enriched food was well tolerated by zebrafish and significantly reduce cholesterol levels in HCD-fed adult zebrafish sometimes below the levels found in control fishes with positive effect on growth of fishes. Additionally, 4% β-sitosterol enriched food was more significant in reducing cholesterol levels in HCD-fed fish than 1% β-sitosterol. The success achieved with 4% β-sitosterol represents the capacity to help maintain healthy cholesterol levels by promoting the reduction of lipid concentrations in the serum in a hypercholesteromic zebrafish model.
Key words: Plant sterols, zebrafish, high cholesterol diet (HCD), hypercholesterolemia.
![]() Corresponding author. Tel.: +8801716717739
Corresponding author. Tel.: +8801716717739
E-mail address: kanizhossain@gmail.com (MK Fatema)
How to cite this article: MK Fatema, Yu Chen and Ge Wei (2015). β-sitosterol reduce cholesterol levels in high cholesterol diet fed zebrafish. International Journal of Natural and Social Sciences, 2(1): 53-65.
INTRODUCTION
Although cholesterol is important to animal body, it has acquired an abhorrent reputation for many years due to heart disease. The excess cholesterol is one of the major risk factors for atherosclerosis that leads to coronary heart disease (CHD) and other cardiovascular conditions such as diseases of the heart and blood vessels. To avoid over accumulation of cholesterol to a harmful level in circulation it is urgent to maintain a healthy cholesterol level. Since mammalian disease models are expensive, the zebrafish system has an opportunity to provide a crucial link between high-throughput in vitro assays and in vivo mammalian disease models (Rubinstein, 2003).
Zebrafish (Danio rerio) is an excellent laboratory model to study lipid metabolism and signaling as it process dietary phospoholipid and cholesterol in a manner analogous to humans and other mammals (Farber et al., 2001). The high-fecundity, rapid development, and low husbandry costs contributed to the zebrafish’s emergence as a premier vertebrate model in biomedicine (Rocke et al., 2009). Feeding adult zebrafish a high cholesterol diet (HCD) resulted in hypercholesteromelia associated with vascular lipid accumulation and profound lipoprotein oxidation, all measured quantitatively in live animals (Stoletov et al, 2009). Zebrafish digestive physiology and lipid metabolism are very similar to that of humans and that treatment of zebrafish with antihyperlipidemic drugs elicits similar responses to their mammalian counterparts (Carten et al., 2009; Stoletov et al., 2009; Farber et al., 2001; Hama et al., 2009). For having all unique advantages, zebrafish was chosen to be the standard test fish in the present study for studying whether a phytosterol named as β-sitosterol can effectively reduce cholesterol levels in HCD-fed adult zebrafish.
Phytosterols are widespread within the plant kingdom, especially among pine trees used in the pulping industry (Rydholm, 1965; Pollak and Kritchevsky, 1981). More than 100 different types of phytosterols (plant sterols) have been identified (Moreau et al., 2002). β -sitosterol is one of the most common ones (Cook et al., 1996). β -sitosterol is found in high concentrations in wood and plant oils, and near similar to cholesterol in terms of chemical structure (Figure 1).
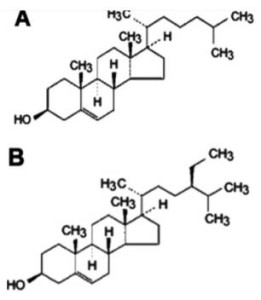
Figure 1
Chemical structures of (A) cholesterol and (B) β –sitosterol (β –sit).
The major phytosterol sources in the human diet are vegetable oils, cereals, fruits, and vegetables (Moreau et al., 2001). Phytosterols are bound to the fibers of the plant and are similar in chemical structure to cholesterol except for the C24 position on the sterol side chain (Young et al., 2004). Some research suggested that because of this structural similarity, β-sitosterol can replace cholesterol in the human body and lowering cholesterol level (Plat et al., 2000). In general, high density lipoproteins (HDL) transport the majority of cholesterol in fish, compared to a predominance of low density lipoprotein (LDL) in mammalian species (Babin and Vernier, 1989). β-Sitosterol is more hydrophobic than cholesterol and it inhibits cholesterol absorption in the intestine (Matsuoka et al., 2008). When the sterol is absorbed in the intestine, it is transported by lipoproteins and incorporated into the cellular membrane (Awad and Fink, 2000). Phytosterols and phytostanols both inhibit the uptake of dietary and biliary cholesterol, and reduce the levels of low density lipoprotein (LDL) and total serum cholesterol. Because the structure of β-sitosterol is similar to that of cholesterol, β-sitosterol takes the place of dietary and biliary cholesterol in micelles produced inthe intestinal lumen (Moreau et al., 2002). This reduces cholesterol absorption in the body. In addition, some researcher reported that β-sitosterol have anti-inflammatory, antipyretic (Bouic, 2002; Gupta et al., 1980), antineoplastic, immune-modulating (Bouic and Lamprecht, 1999), and blood sugar–controlling effects (Ivorra et al., 1988). In herbal therapy β-sitosterol is used especially for benign prostatic hyperplasia (BPH) (Berges et al., 1995). According to Young et al (2004) high dietary or supplemental consumption of phytosterols may be beneficial for women with breast cancer. Therefore it is important to note that more research needs to be conducted to confirm the beneficial and antioxidantal activities of β-sitosterol before recommending it for the treatment or prevention of any form of disease in human. In the present study, β-sitosterol had tested to establish the value of zebrafish in drug discovery once again.
To fully realize the potential of β-sitosterol for reducing cholesterol level in zebrafish, it is important to explore the growth trends of the cholesterol and β-sitosterol treated fishes during the experimental period. As we had a scope to monitor the growth of fishes so we determined differences that occurred in length and weight gain of fishes fed by low and high dose of cholesterol-enriched diet with and without β-sitosterol enriched diet in comparison with control animals that received normal food. In fishes, generally the growth pattern follows the Cube law (Brody, 1945; Lagler, 1952) but the actual relationship may depart from this (Le Cren, 1951) either due to environmental factors or condition of fish. According to King (2007), the morphometric relationships between length and weight can be used to assess the well being of individuals and to determine possible differences between separate unit stocks of the same species. The length-weight relationship also helps to figure out the condition, reproduction history, life history and the general health of fishing species (Nikolsky, 1963; Wootton, 1992; Pauly, 1993; Erkoyuncu, 1995; Avsar, 1998). In addition, length-length relationships are also important in fisheries management for comparative growth studies (Moutopoulos and Stergiou, 2002). Therefore, it looks promising to add length-weight relationship data into HCD-fed zebra fishes to discover the growth trend of high cholesterol and high b-sitosterol treated fishes.
However, there has been hardly any effort so far made to explore the efficacy and effects of β-sitosterol in the HCD-fed zebrafish. Given the importance of all these aspects and to determine the beneficial effect of β-sitosterol in vertebrates, we used zebrafish (Danio rerio) model fed a high cholesterol diet (HCD) along with and without low and high doses of β-sitosterol. The main purpose of the study reported here was to observe the effect of β-sitosterol on cholesterol levels in HCD-fed adult zebrafish. In addition, this study also aims at exploring the pattern of growth of zebrafishes obtained from different feeding strategies during the study period.
MATERIALS AND METHODS
Zebrafish Maintenance and Feeding
All zebrafish studies described in the paper were performed under license from the Government of the Hong Kong SAR and endorsed by the Animal Experimentation Ethics Committee of the Chinese University of Hong Kong. Wild-type (AB) zebrafish embryos were obtained by in vitro fertilization and natural spawning of adult maintained at 28oC on a 14/10 hour light/dark cycle and staged as described by Kimmel et al (1995). Then the larvae were reared with commercial tropical fish food named Otohime until one and half month. The fish were then collected randomly from the mother stock and separately stocked in five separate tanks at the aquarium of the School of Life Sciences, The Chinese University of Hong Kong. Each tank contains 30 adult individual fish (Total 150 individual fish for five treatments).
Treatment with cholesterol and β-sitosterol
Zebrafish were cultured in five different tanks and fed with food containing different doses of cholesterol and β-sitosterol. The control group was fed by commercial tropical fish food named Otohime (0% cholesterol and 0% β-sitosterolin food) which was expressed as “control diet” (treatment 1, n=30). The four experimental diets were prepared by adding 1% cholesterol only (treatment 2, n=30), 1% cholesterol + 1% β-sitosterol (treatment 3, n=30), 4% cholesterol only (treatment 4, n=30) and 4% cholesterol + 4% β-sitosterol (treatment 5, n=30), respectively into the control diet. The food containing 1% and 4% cholesterol was expressed as high cholesterol diet (HCD); HCD was made by soaking Otohime B1(crude protein 53%, crude fat 8%, crude fibre 3%, crude ash 16%, calcium 2.30%, phosphorus 1.5%) in a diethyl ether solution of cholesterol (Catalog number C-8503, Sigma). Similarly, 1% cholesterol + 1% β-sitosterol diet (final concentration) and 4% cholesterol + 4% β-sitosterol (final concentration) was made by soaking Otohime B1 in a diethyl ether solution of cholesterol and β-sitosterol (Ningbo Distant Chemicals Co. Ltd, Ningbo, China). Each group (n=30) consumed the cholesterol and β-sitosterol treated diet (20mg/day per fish) according to above plan for 5 weeks.
Measurement of length-weight of fish
After feeding for five weeks, all fish from each treatment (Total=150 individual fish from five treatment) were measured in the laboratory to monitor whether there is any differences in length and weight gain of fishes in different environmental condition treated with different cholesterol-enriched diet along with or without β-sitosterol enriched diet. The standard length (SL), total length (TL), and fork length (FL) of all individuals sampled were measured to the nearest 1 mm and body weight (BW) to the nearest 0.001 mg. The statistical significance level of r2 was estimated and the parameters a and b were estimated by least square linear regressions performed on the transformed equation, log BW = log a + b × log SL, where BW is the body weight (mg) and SL is the standard length (mm). When the b value in length-weight relationship was equal to or did not show statistically significant deviation from 3, the growth was isometric, whereas the positive or negative allometric growth occurred when the b value deviated significantly from 3(Ricker, 1975; Erkoyuncu, 1995). All statistical analyses were evaluated at P<0.05 significance level.
Determination of blood cholesterol
To perform blood cholesterol study fishes were fasted for 24 hours before blood collection to avoid food intake interference. The analyses of samples were done in the same day of collection of blood from five treatments. Blood samples were handled carefully as zebrafish blood can incur hemolysis very easily. For total cholesterol analyses, 10 μL of serum was used. These analyses were performed on pooled samples of 10 zebrafish per sample. After feeding for 5 weeks, blood (2 μL) was drawn from fish tail vein (total 10 zebra fish of each treatment) combined with 5 μL of PBS-EDTA (final concentration, 1 mM) and then collected in EDTA-treated tubes as described by Jin et al (2011). The plasma was obtained by centrifugation. Plasma total cholesterol (TC) was determined using enzymatic kits from Stanbio Laboratories (Boerne, TX, USA) and UV-1601 (UV visible Spectrophotomter, Shimadzu, 500 nm).
Determination of tissue cholesterol
Cholesterol levels in body fluids were measured by isolating them from the adult fish (total 10 zebra fish in each treatment) from which abdomens were removed and bodies were centrifuged to pellet tissue debris, and supernatants were used to extract total lipid and measure cholesterol using a GLC equipped with a flame-ionization detector. In brief, total lipids were extracted from ~100 mg body tissue sample with addition of 1 mg of stigmastanol as an internal standard, using 15 mL chloroform/methanol (2:1, v/v) and 3 mL saline. After the mild hydrolysis with 5 mL NaOH in 90% ethanol at 90oC, esterified cholesterol was converted to free cholesterol. Total cholesterol was then extracted with 1 mL of water and 6 mL of cyclohexane. After centrifugation, the extract was evaporated to dryness under a gentle stream of nitrogen. Cholesterol was converted to its trimethyl-silyl (TMS)-ether derivative by a commercial TMS reagent (dry pyridine/hexamethyldisila-zane/trichlorosilane, 9:3:1 v/v/v, Sil-A reagent; Sigma, St. Louis, MO, USA). Analysis of the cholesterol TMS-ether derivative in hexane was performed in a fused silica capillary column (SACTM-5, 30 m ´ 0.25 mm, id; Supelco, Bel-lefonte, PA, USA) in a Shimadzu GC-14B GLC equipped with a flame-ionization detector (Shimadzu, Tokyo, Japan).
Statistical analysis
Data were subjected to one way analysis of variance (ANOVA) followed by post hoc multiple comparisons using Tukey test to determine the significant differences among five treatments. The results presented are the mean with standard errors. The minimum accepted P value for significance is 0.05.
RESULTS AND DISCUSSION
High cholesterol diets were fed to adult zebrafish for 5 weeks to examine whether zebrafish are sensitive to high-cholesterol diet feeding and at the same time β-sitosterol were added in food to examine whether it can reduce the level of cholesterol. We measured tissue cholesterol levels and blood cholesterol levels from each treatment to render the results more reliable.
Food intake
Visual observation revealed no marked abnormalities or major differences in feeding behavior between the five treatments. Adding cholesterol and b-sitosterol into food did not affect survival of fish, their swimming pattern nor apparent food intake. No mortality was occurred during experimental period.
Fish length and body weight relationship
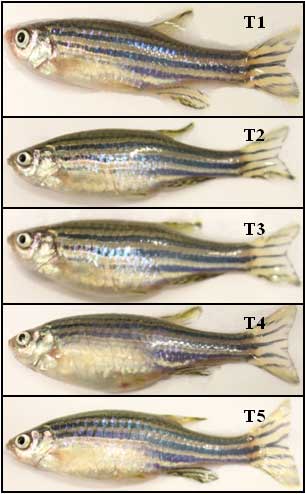
After feeding fishes with cholesterol and b-sitosterol-enriched diet for five weeks, the body weight (mg), total length (TL), standard length (SL) and fork length (FL) of all individuals in five treatment groups were measured to know the effects of cholesterol- and b-sitosterol enriched diet on fish growth trend. We found that the fish fed with cholesterol and b-sitosterol enriched diet had an enlarged belly compared to control fish (Figure 2).
Figure 2
Female fish (confirmed by dissection) fed a control diet, HCD and β-sitosterol diet in five treatments. Adult zebrafish (both male and female) were fed a cholesterol-enriched (HCD) or β-sitosterol-enriched diet for 5 weeks.
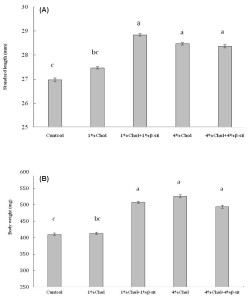
Standard length (SL) is mainly used in systematic studies. When making comparisons between populations, it is essential to use standard measures for all populations so that the results will be more reliable. That is why standard length (SL) was used for the present study because it is a universal standard for measurement of length of fishes. Standard length (mm) and body weight (mg) were ranged from 19-30 mm and 272.9-751.3 mg for control fish (treatment 1), 25-30 mm & 285.7-681.2 mg for treatment 2, 27-33 mm & 388.9-804.7 mg for treatment 3, 26-31 mm & 321.8-757.3 mg for treatment 4 and 26-33 mm & 289.8-775.0 mg for treatment 5, respectively. The lowest value of standard length was measured as 25 mm in 1% cholesterol treatment while the highest value was 33 mm in both 1% cholesterol + 1% b-sitosterol and 4% cholesterol + 4% b-sitosterol mixed diet treatment, respectively. The lowest value of body weight was recorded as 285.7 mg in 1% cholesterol treatment while highest value was 775.0 mg in 4% cholesterol + 4% b-sitosterol mixed diet treatment. The result showed that standard length and body weight was increased significantly when b-sitosterol and cholesterol in diet increased from 0 to 1% for treatment 3 and 0 to 4% for treatment 4 and 5, respectively, where cholesterol diet increased to 0 to 1% in treatment 2 showed a little increase in length and body weight during the experimental period but this was not significant. Both the high cholesterol and high b-sitosterol feeding group showed increased length and body weight compared to the normal group after 5 weeks of feeding with cholesterol and β-sitosterol in the normal diet (Figure 3). The length-weight relationship of five treatment group presented in figure 4 and table 1, showed that linear regressions on log transformed data were not significant for treatment 2 and 3 but treatment 4 and 5 showed significant data with r2> 0.7~0.8. There have been a few studies available on length-weight relationship of HCD treated fish. According to Jin et al (2011), the body weight of the HCD group was increased by 11% compared to the normal group after 5 weeks of feeding 4% cholesterol in the normal diet. They also reported that in contrast to the increase, the height was significantly decreased (up to 4%) compared to the normal group (P < 0.002). The weight-to-height (in mg/cm) ratio was increased 16% in the HCD group compared to the normal group. They suggested that HCD might contribute to an increase in body weight, rather than height. In our present study, the length-weight relationship of five treatment group showed that linear regressions on log transformed data were not significant for treatment 2 and 3 but treatment 4 and 5 showed significant b value with r2> 0.7~0.8 (Figure 5 and table 1). The b value in the length-weight relationship of fish can be used as an indicator of food intake and growth pattern, and may differ according to such biotic and abiotic factors as water temperature, food availability and habitat type (Wootton, RS, 1992; Avsar, 1998). However, adequate feeding and gonad development increases fish weight and b values (Nikolsky, 1963; Arslan, 2003). In present study, the calculated allometric coefficient b ranged from a minimum of 2.4009 for treatment 2 (1% cholesterol diet), to a maximum of 4.5468 for treatment 4 (4% Cholesterol diet), with an average value of 3.141. Our finding suggest that 1% cholesterol + 1% β-sitosterol, 4% cholesterol enriched and 4% cholesterol & 4% β-sitosterol mixed enriched diet contribute positively to fish growth by increasing fish length and body weight. Although r2> 0.5 for treatment 3 but b value recorded as 2.9373 which is more or less closer to 3 indicates isometric growth of most of the fishes in this treatment. The fishes of these three treatments feed sufficiently and showed higher b value indicated isometric and positive allometric growth. On the other hand, 1% cholesterol enriched taken fishes feed insufficiently and display low b values during the experimental period that demonstrated a negative allometric growth. Most importantly, fishes successfully took cholesterol- and b-sitosterol enriched diet without any mortality or difficulty.
Figure 3
Effects of cholesterol- and β-sitosterol enriched diet on (A) standard length and (B) body weight of HCD-fed adult zebrafish. Zebrafish were fed a cholesterol-enriched (HCD) or β-sitosterol-enriched or control diet for 5 weeks. Data represents mean of 30 individual fish sample for each treatment with standard error (mean±SE) and different letters above the bars represent significant differences among means of different treatment (P< 0.05).
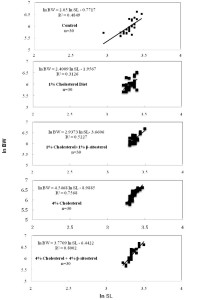
Figure 4
Effects of cholesterol- and β-sitosterol enriched diet on length-weight relationship (In BW = In a + b × In SL) of pooled zebrafish for five treatment (n=30). Zebrafish were fed a cholesterol-enriched (HCD) or β-sitosterol-enriched or control diet for 5 weeks.
Table 1
Length-weight relationships equation (In BW = Ina + b × In SL) of zebrafish for five treatments.
| No | Treatment | Equation | Co-efficient of determination (r2) |
| 1. | Control | In BW = 2.05 In SL – 0.7717 | 0.4849 |
| 2. | 1% Cholesterol diet | In BW = 2.4009 In SL – 1.9567 | 0.3126 |
| 3. | 1% Chol+1% β-sit | In BW = 2.9373 In SL- 3.6606 | 0.5227 |
| 4. | 4% Cholesterol diet | In BW = 4.5468 In SL – 8.9885 | 0.7568 |
| 5. | 4% Chol+4% β-sit | In BW = 3.7709 In SL – 6.4422 | 0.8002 |
Data represents mean of 30 individual fish sample for each treatment. Zebrafish were fed a cholesterol-enriched (HCD) or β-sitosterol-enriched or control diet for 5 weeks.
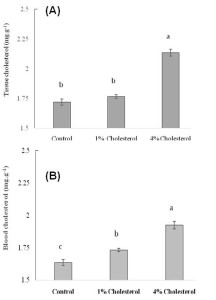
Figure 5
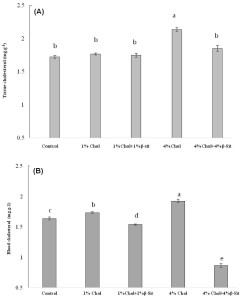
Effect of different doses of chloesterol on (A) tissue cholesterol and (B) blood cholesterol levels in HCD-fed adult zebrafish. Zebrafish were fed a cholesterol-enriched (HCD) or control diet for 5 weeks. Data represents mean of 10 individual fish sample for each treatment with standard error (mean±SE) and different letters above the bars represent significant differences among means of different treatment (P< 0.05).
Effect of cholesterol in diet on fish tissue and blood cholesterol
The plasma total cholesterol levels of the HCD group were elevated in a dose-dependent manner when cholesterol in diet increased from 0 to 4% than the control group of fishes (Figure 5). In general, cholesterol in tissue and blood increased sharply when dietary cholesterol increased from 0 to 4%. However, we found that when dietary cholesterol increased from 0 to 1% in treatment 2, total cholesterol was significantly elevated as 1.7307 mg/dl for blood cholesterol where tissue cholesterol elevated as 1.7663 mg/g but was not significant. In contrast, when 4% cholesterol was added to food in treatment 4, total cholesterol was significantly elevated one and half fold higher in zebrafish than the control group (Figure 5). Total cholesterol levels were raised significantly up to 2.1363 mg/g and 1.923 mg/dl in tissue and blood cholesterol study, respectively, when 4% cholesterol added in diet in treatment 2 and 4. This result indicates that increased cholesterol uptake lead to significant elevations in cholesterol levels in zebrafish comparable with hypercholesterolemia as well as zebrafish were hyper-responsive to high dose of dietary cholesterol. Research evidence provides information on oxidized lipoprotein that plays an important role in atherogenesis in humans and in animal models (Tsimikas et al., 2005; Glass and Witztum, 2001).
Figure 6
Effect of different doses of β-sitosterol on (A) tissue cholesterol and (B) blood cholesterol levels in HCD-fed adult zebrafish. Zebrafish were fed a cholesterol-enriched (HCD) or β-sitosterol-enriched or control diet for 5 weeks. Data represents mean of 10 individual fish sample for each treatment with standard error (mean±SE) and different letters above the bars represent significant differences among means of different treatment (P< 0.05).
By feeding HCD to zebrafish, we observed hypercholesterolemia in adult zebrafish that is likely involved in early atherogenesis. This finding indicates that plasma blood glucose may increase due to increase uptake of cholesterol. However, these results suggest a similar trend of experimental conditions used in experiments with zebrafish reported by other researchers (Stoletov et al., 2009; Fang et al., 2010 and Wen et al., 2004). Treatment with HCD feed direct the performance to significant elevations in cholesterol levels in zebrafish fluids, comparable with hypercholesterolemia reported for adult zebrafish fed a HCD (Stoletov et al., 2009). Stoletov et al (2009) observed hypercholesterolemia, lipoprotein oxidation and fatty streak formation by feeding HCD to zebrafish. Fang et al (2010) suggested that HCD feeding initiates zebrafish larvae profound lipid oxidation and that resulting oxidized lipid species closely match those found in mammalian atherosclerosis and active important proatherogenic mechanisms. Thus for studying of pathological process important in early atherogenesis, zebrafish is an attractive experimental model organism.
Hypolipidemic effect of β-sitosterol in zebrafish
Although there have been many reports concerning the oxidized cholesterol esters and phospholipids in zebrafish (Fang et al., 2010) relatively few articles have been published regarding the potency of b-sitosterol on reduction of cholesterol level in a HCD-fed zebrafish. The present study demonstrates the cholesterol-lowering activity of 1% and 4% b-sitosterol and blood & plasma cholesterol concentrations of HCD-fed adult zebrafish. Normal feed fed zebrafish was used as control to examine whether treatment with b-sitosteorl effects on HCD-fed zebrafish. To study the effect of β-sitosterol on cholesterol diet, we initially added 1% β-sitosterol to 1% cholesterol (final concentration, 1% [wt/wt]) in treatment 3 and 4% β-sitosterol to 4% cholesterol (final concentration, 4% [wt/wt]) in treatment 5. In general, a dose-dependent decrease of cholesterol concentration was seen in zebrafish with the increased in β-sitosterol in diets. Cholesterol in tissue and blood decreased sharply when dietary β-sitosterol increased from 0 to 4% and found lower cholesterol level that was below the levels found in control fishes. However, we found that when dietary β-sitosterol increased from 0 to 1% in treatment 3, total blood cholesterol was significantly decreased as 1.5384 mg/dl and total tissue cholesterol was decreased as 1.7467 mg/g, which was not significant. In contrast, total cholesterol level was significantly decreased when dietary β-sitosterol increased from 0 to 4% for both tissue and blood cholesterol study (Figure 6). When 4% β-sitosterol added to food in treatment 5, total blood cholesterol was significantly decreased as 0.8653 mg/dl than those fishes who received HCD-feed only as well as that was one fold lower than the control group. Tissue cholesterol analyses also support the significant decrease trend of cholesterol level after adding 4% β-sitosterol diet in food. This result suggested a clear evidence of efficacy of β-sitosterol in reducing cholesterol level in HCD-fed zebrafish. Both tissue and blood cholesterol study confirmed the result but blood cholesterol study showed sharper and clearer result of the efficacy of β-sitosterol on cholesterol level than tissue cholesterol study.
The present investigation demonstrated clearly that 4% β-sitosterol effectively reduced cholesterol level in HCD-fed zebrafish with positive fish growth. b-sitosterol is one of the major phytosterols in higher plants. The phytosterols are very stable and intense processes (such as boiling, neutralization, bleaching, and deodorization) do not affect the phytosterol content of vegetables and fruits (Normen et al., 1999, Bortolomeazzi et al., 2000). Moreau et al (2002) reported that due to poor solubility and bioavailability of phytosterols, the serum cholesterol–lowering effect of phytosterols is not consistent, and high dosages (up to 25 to 50 g/d for human) are required for efficacy. We observed significant positive effect of b-sitosterol in reducing of cholesterol level when we used high dose (final concentration, 4% [wt/wt]) of b-sitosterol in food in treatment 5 to zebrafish as compared to low dose in treatment 3. Therefore, our results with zebrafish suggest a similar trend of experimental results compared with above studies.
Previous studies have shown b-sitosterol to be effective in reducing plasma steroid concentrations in short-term exposures in goldfish (MacLatchy and Van Der Kraak, 1995) and trout (Gilman et al., 2003). In the present study, the five-week treatment with 1% and 4% b-sitosterol significantly reduces cholesterol level among treatment groups. The plasma total cholesterol level was markedly reduced in 4% b-sitosterol fed group than the HCD fed group. Furthermore, the blood cholesterol level showed clearer evidence of significantly decreasing cholesterol level compared to the HCD-group and below the levels found in control fishes. The above result suggests that 1% and 4% b-sitosterol was very effective in reducing cholesterol levels in HCD-fed adult zebrafish, indicating a higher potency of the b-sitosterol treatment. More specifically, 4% β-sitosterol was highly significant in reducing cholesterol levels in HCD-fed fish than 1% β-sitosterol, demonstrated a potential mode of action of b-sitosterol on cholesterol status. Lowering cholesterol by b-sitosterol slows the fatty buildup in the arteries, and in some cases can help reduce the buildup already there. This finding also suggested that a higher dose of b-sitosterol could effectively work on target cholesterol without affecting food intake, survival and swimming pattern of fishes.
Research reveals little information regarding direct mechanism of b-sitosterol on reducing cholesterol level. Inverse correlations between plant sterol intake and cholesterol absorption have been reported in humans (Ellegard et al., 2000), hamsters (Ntanios and Jones, 1999; Carr et al., 2002), and rabbits (Ntanios and Jones, 1999). The exact mechanism by which b-sitosterol inhibit cholesterol absorption is not fully understood. Carr and Jesch (2006) proposed several mechanisms of cholesterol absorption by b-sitosterol, including competition with cholesterol for solubilization in micelles within the intestinal lumen, interaction with digestive enzymes, co-crystallization with cholesterol to form insoluble crystals, and regulation of intestinal transporters of cholesterol etc. The major mechanism of action has long been assumed to be the competition between cholesterol and plant sterols for micelle solubilization (Ikeda and Sugano, 1983) although direct evidence is surprisingly limited. Gilman et al (2003) reported that goldfish exposed to b-sitosterol in vivo for short periods experience changes in plasma cholesterol and lipoprotein dynamics as both HDL and total cholesterol concentrations are reduced. We determined that treatments of HCD-fed zebrafish with 4% b-sitosterol reduced cholesterol levels as much as one fold lower than in zebrafish that received control diet and no other treatment. Fish having a body temperature that varies with the temperature of its surroundings preferentially use lipids rather than carbohydrates as an energy source and would be classified, using standards applied to mammals, as mildly hyperlipidemic and hypercholesterolemic, even when consuming their normal diet (Babin and Vernier, 1989). Treatments with 1% and 4% b-sitosterol were able to reduce normal zebrafish hypercholesterolemia with successful functioning of zebrafish normal life cycle.
CONCLUSION
The findings of the present work provide valuable information and enrich knowledge on b-sitosterol exposure to fish that can altered cholesterol dynamics and decrease cholesterol levels of HCD-fed adult zebrafish. 1% and 4% HCD-fed resulted increases in cholesterol levels in blood and tissue plasma of adult zebrafish. At the same time, 1% and 4% b-sitosterol can effectively reduces cholesterol levels in HCD-fed fish and sometimes below the levels found in control fishes as well as had positive effect on growth of fishes. More specifically, 4% β-sitosterol was highly significant in reducing cholesterol levels in HCD-fed fish than 1% β-sitosterol, demonstrated a potential mode of action of b-sitosterol on cholesterol status. Therefore, these results suggest that 4% b-sitosterol enriched diet can be more suitable and effective to use in the hypercholesterolemic zebrafish model study. Additional studies are necessary to examine the activity of b-sitosterol to compare their physiologic roles in the animal model like human.
REFERENCES
Arslan M (2003). Çoruh Havzası Anuri ve Cenkerçaylarında yasayan alabalık, Salmo trutta Linneaus 1766, populasyonları üzerine arastırmalar. Ph.D.Thesis. Erzurum: Atatürk University.
Avsar D (1998). Fisheries biology and population dynamics. University of Cukurova, Faculty of Fisheries, Adana, Turkey, p. 303 (in Turkish).
Awad (AB) and Fink CS (2000). Phytosterols as Anticancer Dietary Components: Evidence and Mechanism of Action. Journal of Nutrition,130 (9): 2127–2130.
Babin PJ and Vernier JM (1989). Plasma lipoproteins in fish. Journal of Lipid Research, 30: 467–489.
Berges RR, Windeler J, Trampisch HJ and Senge T (1995). Randomised, placebo-controlled, double-blind clinical trial of beta-sitosterol in patients with benign prostatic hyperplasia. Beta-sitosterol Study Group. Lancet, 345 (8964): 1529–1532.
Bortolomeazzi R, De Zan M, Pizzale L and Conte LS (2000). Identification of new steroidal hydrocarbons in refined oils and the role of hydroxy sterols as possible precursors. Journal of Agricultural and Food Chemistry, 48: 1101–1105.
Bouic PJ and Lamprecht JH (1999). Plant sterols and sterolins: areview of their immune-modulating properties. Alternative Medicine Review, 4: 170–177.
Bouic PJ (2002). Sterols and sterolins: new drugs for the immunesystem? Drug Discovery Today, 7: 775–778.
Brody S (1945). Bioenergetivs and Growth. Reinhold Publishing Corporation, New York. 1023p.
Carr TP and Jesch ED (2006). Food ingredients that reduce cholesterol absorption. Advances in Food and Nutrition Research, 51:165-204.
Carr TP, Cornelison RM, Illston BJ, Stuefer-Powell CL and Gallaher DD (2002). Plant sterols alter bile acid metabolism and reduce cholesterol absorption in hamsters fed a beef-based diet. Nutrition Research, 22: 745 – 54.
Carten JD and Farber SA (2009). A new model system swims into focus: using the zebrafish to visualize intestinal metabolism in vivo. Clinical Lipidology, 4(4):501–515.
Cook DL, LaFleur L, Parrish A, Jones J and Hoy D (1996). Characterization of plant sterols in a selected group of US pulp and paper mills. In: Fifth IEAWQ symposium preprint of forest industry waste water. Vancouver, Canada, pp. 1–8.
Ellegard L, Bosaeus I and Andersson H (2000). Will recommended changes in fat and fibre intake affect cholesterol absorption and sterol excretion? An ileostomy study. European Journal of Clinical Nutrition, 54:306-13.
Erkoyuncu I (1995). Fisheries biology and population dynamics. University of Ondokuz Mayis, Faculty of Fisheries, Sinop, Turkey, p. 256 (in Turkish).
Fang L, Harkewicz R, Hartvigsen K, Wiesner P, Choi SH, Almazan F, Pattison J, Deer E, Sayaphupha T, Dennis EA, Witztum JL, Tsimikas S and Miller YI (2010). Oxidized cholesteryl Esters and phospholipids in zebrafish larvae fed a high cholesterol diet. The Journal of Biological Chemistry, 285 (42): 32343-32351.
Farber SA, Pack M, HO SY, Johnson ID, Wagner DS, Dosch R, Mullins MC, Hendrickson HS, Hendrickson EK and Halpern ME (2001). Genetic analysis of digestive physiology using fluorescent phospholipid reporters. Science, 292: 1385-1388.
Gilman CI, Leusch FDL,.Breckenridge WC and MacLatchy DL (2003). Effects of a phytosterol mixture on male fish plasma lipoprotein fractions and testis P450scc activity. General and Comparative Endocrinology, 130: 172–184.
Glass CK and Witztum JL (2001). Atherosclerosis. The road ahead. Cell, 104: 503-516.
Gupta MB, Nath R, Srivastava N, Shanker K, Kishor K and Bhargava KP (1980). Anti-inflammatory and antipyretic activities of beta-sitosterol. Planta Medica, 39: 157–163.
Hama K, Provost E, Baranowski TC, Rubinstein AL, Anderson JL, Leach SD and Farber SA (2009). In vivo imaging of zebrafish digestive organ function using multiple quenched fluorescent reporters. American journal of physiology. Gastrointestinal and liver physiology, 296(2):G445–G453.
Ikeda I and Sugano M (1983). Some aspects of mechanism of inhibition of cholesterol absorption by b-sitosterol. Biochim. Biophys. Acta. 732:651 – 8.
Ivorra MD, D’Ocon MP, Paya M and Villar A (1988). Antihyperglycemicand insulin-releasing effects of beta-sitosterol 3-beta-D-glucoside andits aglycone, beta-sitosterol. Archives internationales de pharmacodynamie et de thérapie, 296: 224–231.
Jin S and Cho KH (2011). Water extracts of cinnamon and clove exhibits potent inhibition of protein glycation and anti-atherosclerotic activity in vitro and in vivo hypolipidemic activity in zebrafish. Food and Chemical Toxicology, 49(7):1521–1529.
Jin S, Hong JH, Jung SH and Cho KH (2011). Turmeric and Laurel aqueous extracts exhibit In Vitro anti-atherosclerotic activity and In Vivo hypolipidemic effects in a zebrafish model. Journal of Medicinal Food, 14 (3):247-256.
Kimmel, C.B., W.W. Ballard, S.R. Kimmel, B. Ullmann and T.F. Schilling. 1995. Stages of embryonic development of the zebrafish. Developmental Dynamics, 203 (3):253-310.
King, M. 2007. Fisheries biology, assessment and management. Second Edition. Blackwell Scientific Publications, Oxford: 1-381.
Lagler KF (1952). Freshwater fishery biology. W.M.C. Brown Company, Dubyque. Iowa.
Le Cren ED (1951). The length-weight relationships and seasonal cycle in gonad weight and condition in the perch (Perca fluviatilis). Journal of Animal Ecology, 20:201-219.
MacLatchy D and Van Der Kraak G (1995). Effects of the phytoestrogen b-sitosterol on the reproductive endocrine status of goldfish. Toxicology and Applied Pharmacology, 134: 305–312.
Matsuoka K, Nakazawa T, Nakamura A, Honda C, Endo K and Tsukada M (2008). Study of thermodynamic parameters for solubilization of plant sterol and stanol in bile salt micelles. Chemistry and Physics of Lipids, 154 (2): 87–93.
Moreau RA, Singh V and Hicks KB (2001). Comparison of oil and phytosterol levels in germplasm accessions of corn, teosinte, and Job’s tears. Journal of Agricultural and Food Chemistry, 49: 3793–3795.
Moreau RA, Whitaker BD and Hicks KB (2002). Phytosterols, phytostanols, and their conjugates in foods: structural diversity, quantitative analysis, and health-promoting uses. Progressive Lipid Research, 41: 457–500.
Moutopoulos DK and Stergiou KI (2002). Length-weight and length-length relationships of fish species from Aegean Sea (Greece). Journal of Applied Ichthyology, 18: 200-203.
Nikolsky GW (1963). The ecology fishes. Academic Press, London and New York. 352 pp.
Normen L, Johnsson M, Andersson H, van Gameren Y and Dutta P (1999). Plant sterols in vegetables and fruits commonly consumed in Sweden. European Journal of Nutrition, 38: 84–89.
Ntanios FY and Jones PJH (1999). Dietary sitostanol reciprocally influences cholesterol absorption and biosynthesis in hamsters and rabbits. Atherosclerosis, 143:341-51.
Pauly D (1993). Fishbyte section editorial. Naga, the ICLARM Quarterly, 16: 26.
Plat J, Kerckhoffs DA and Mensink RP (2000). Therapeutic potential of plant sterols and stanols. Current Opinion in Lipidology, 11:571-576.
Pollak OJ and D Kritchevsky (1981). Sitosterol. In: Clarkson TB, Kritchevsky D, Pollack OJ (Eds.), Monographs on atherosclerosis, vol. 10. Krager, New York, pp. 4–22.
Ricker WE (1975). Computation and interpretation of biological statistics of fish populations. Bulletin Fisheries Research Board of Canada, 191: 203-233.
Rocke J, Lees J, Packham I and Chico T (2009). The zebrafish as a novel tool for cardiovascular drug discovery. Recent Patents on Cardiovascular Drug Discovery, 4(1):1–5.
Rubinstein AL (2003). Zebrafish: From disease modeling to drug discovery. Current Opinion in Drug Discovery & Development, 6(2):218-223.
Rydholm SA (1965). Pulping process. Wiley and Sons, New York, New York.
Stoletov K, Fang L, Choi SH, Hartvigsen K, Hansen LF, Hall C, Pattison J, Juliano J, Miller ER, Almazan F, Crosier P, Witztum JL, Klemke RL and Miller YI (2009). Vascular lipid accumulation, lipoprotein oxidation and macrophage lipid uptake in hypercholesterlemic zebrafish. Circulation Research, 104: 952-960.
Tsimikas S, Brilakis ES, Miller ER, McConnell JP, Lennon RJ, Kornman KS, Witztum JL and Berger PB (2005). Oxidized phospholipids, Lp(a) lipoprotein, and coronary artery disease. New England Journal of Medicine, 353:46-57.
Wen LS, Eng K, Lee J and McGrath P (2004). Use of a monoclonal antibody specific for activated endothelial cells to quantitate angiogenesis in vivo in zebrafish after drug treatment. Angiogenesis, 7 (3): 243-253.
Wootton JT (1992). Indirect effect, prey susceptibility, and habitat selection: impacts of birds on limpets and algae. Ecology, 73(3): 981-991.
Wootton RS (1992). Fish Ecology. Printed in Great Britain by Thomson Litho Ltd., Scotland.
Young HJ, Clausen LM, Allred KF, Almada AL and Helferich WG (2004). β-Sitosterol, β-Sitosterol Glucoside, and a Mixture of β-Sitosterol and β-Sitosterol Glucoside Modulate the Growth of Estrogen-Responsive Breast Cancer Cells In Vitro and in Ovariectomized Athymic Mice. Journal of Nutrition, 1145-1151.