Epidemiology of PPR in goat in Kushtia district, Bangladesh
Volume 2, Issue 1, February 2015, Pages 107–112
Sharifuzzaman1![]()
![]() , Md. Abdullah Al Noman2, M.A. Jalil3
, Md. Abdullah Al Noman2, M.A. Jalil3![]() , Md. Hasibul Haque4
, Md. Hasibul Haque4![]() , Zinat Shahjada1, Abdullah-Al-Motin1
, Zinat Shahjada1, Abdullah-Al-Motin1
1Faculty of Animal Science and Veterinary Medicine, Patuakhali Science and Technology University, Babugonj, Barisal-8210, Bangladesh
2Faculty of Veterinary Medicine, Chittagong Veterinary and Animal Sciences University, Khulshi, Chittagong, Bangladesh
3Veterinary surgeon, District veterinary Hospital, Dinajpur-5200, Bangladesh
4Department of Animal Husbandry and Veterinary Science, University of Rajshahi, Rajshahi-6205, Bangladesh
| ABSTRACT | Get Full Text PDF |
The study was conducted at upazila Veterinary Hospital, Kushtia Sadar, Kushtia in the year 2008. A total number of 280 diseased goats were examined of which 35 (12.50%) Peste Des Petits Ruminants (PPR) cases were diagnosed on the basis of clinical sign, gross and pathological lesion. Dullness and depression, rough hair coat, dyspnoea, diarrhea, swollen and erosion of lip and occulonasal discharge were the common findings during clinical examination of PPR patients. About 7-12 months age group of goat was found more prone (20.87%) to PPR than other. Black Bengal goat was more susceptible (13.47%) than Jamunapari (8.00%). Non vaccinated goats were more prone (14.91%) to PPR infection than vaccinated goats (1.92%). Lymphocytes percentages were started to decline (40%) and neutrophils percentages were started to increase (58%) from day 3 of clinical manifestation of PPR, which have got reached 28% of lymphocytes and 66% of neutrophils on day 6 of PPR. The percentage of response to treatment with parenteral (I/M) sulphadimidin was higher (56.52%) than parenteral oxytetracycline (41.66%) along with symptomatic treatment.
Key words: Peste Des Petits Ruminants (PPR), goat, Kushtia , Bangladesh.
![]() Corresponding author. Tel.: +8801717513544
Corresponding author. Tel.: +8801717513544
E-mail address: sharif.edu.bd@gmail.com (Sharifuzzaman)
How to cite this article: Sharifuzzaman, MAA Noman, MA Jalil, MH Haque, Z Shahjada and AA Motin (2015). Epidemiology of PPR in goat in Kushtia district, Bangladesh. International Journal of Natural and Social Sciences, 2(1): 107-112.
INTRODUCTION
The livestock population of Bangladesh is 58.96 million, of which goat population is about 33.5 million which contribute 3.96% in GDP (Gross Domestic Product) (Alam, 2002). Traditionally goat rearing is an integral part of` mixed farming in Bangladesh and the contribution of goat to the national economy is valued for high quality meat, milk, skin also for poverty alleviation, income generation, creation of employment opportunity and food production (Debnath,1995). Different types of infectious diseases (PPR, pneumonia, goat pox, enterotoxaemia, mastitis, tetanus, ringworm etc) are important problems in goat rearing in our country. Among the diseases that have a great significance and economic value in goat rearing, PPR (Peste Des Petits Ruminants) is the most devastating disease. PPR (goat plague, stomatitis syndrome) is an infectious, highly contagious viral disease of small ruminants particularly goat and sheep (Roeder et al, 1994). The virus is antigenically related to rinderpest virus which infects cattle and other large ruminants (Barrett, 1994). It is the member of the genus Morbillivirus which also includes measles, canine distemper and viruses of marine mammals in the family Paramyxoviridae (Barrett et al., 1994). It is also known as pseudo rinderpest of small ruminants, pest of small ruminants, pest of goats, kata, stomatitis, pneumoenteritis syndrome, contagious pustular stomatitis and pneumoenteritis complex.
PPR was first recorded as clinical entity in the Ivory Cost of West Africa in 1942 (Samad, 1996). It is an acute or sub acute viral disease of goats characterized by high fever (104-106°F), necrotic stomatitis, gastroenteritis, pneumonia which has different epidemic pattern and endemic in nature found throughout the world; East and West Africa, the middle East, Senegal, Ghana, Sudan, Indian sub-continent including Bangladesh (Roeder and Obi, 1999). Among the South Asian countries, PPR virus was first recorded in India from the southern state of Tamilnadu in 1947 and it continued to be present in the southern state of Andrapradesh and Karnataka (Debnath, 1995). Transmission occur by close contact, inhalation of aerosol produced by sneezing and coughing of infected animals, direct contact with ocular, nasal, oral secretion, faeces, fomites such as bedding, water and feed troughs (Ozkul et al., 2002).
In Bangladesh rinderpest like disease i.e. PPR was first noticed by FAO expert team while visiting western part of Bangladesh in 1993. PPR was detected by sample taken from the sick goat and then spread an epidemic fashion throughout the country (Debnath, 1995). By the year 1995, it is assumed that almost 75 percent of the districts in Bangladesh are affected with PPR.
Poverty alleviation through goat rearing, the program of Bangladesh government will be null and void if the PPR disease will not be control through proper vaccination and taking of other preventive measure. Considering all aspects described, the present study, therefore, undertaken to study the epidemiology of PPR in goat in the study area and determining the efficacy of symptomatic treatment in PPR infected goats.
MATERIALS AND METHODS
The study was conducted at upazila veterinary hospital, Kushtia Sador, in the year, 2008. Information about the disease and clinical signs exhibited by the animal during illness were recorded in details provided by the owner.
Haematological examination
Five blood samples were collected randomly from day 3, 3, 4, 5 and 8 days of infection. Differential leukocytes count (DLC) were performed by smearing blood film with Wright’s stain and counted under light microscope at Paribarick Sastho Clinic, Kumarkhali, Kushtia. The percentage of lymphocyte and neutrophil were recorded.
Diagnosis of PPR
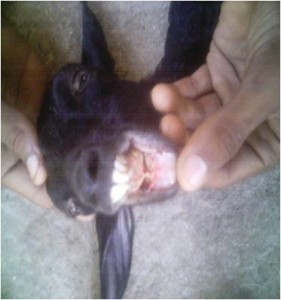
Diagnosis was made by means of history, clinical signs, physical examination and sometime hematological findings and postmortem examination. Gross photographs of the sick goats showing different types of clinical signs were taken (Figure 1-4).
Treatment
Symptomatic treatment given to all the PPR patients were divided into 2 groups and response to the treatment was recorded. Group -1 was treated with Diadin (Sulphadimidine-Na) @ 0.5ml (166.5mg)/kg body weight I/M at first day followed by half of the initial day for next 4 days, Dellergen (Promethazin HCL) @ 0.2 ml (5mg)/kg body weight I/M daily for 3 days and Renalyte (Oral Rehydrated Solution) @ 0.5 liter (0.625gm)/adult goat orally at every 6hours interval for 5 days.
Group -2 was treated with Renamycin-100 (Oxytetracycline) @ 0.1 ml (1omg)/kg body weight I/M daily for 5 days, 2 Dellergen (Promethazine Hcl) @ 0.1 ml(5 ml)/kg body weight I/M daily for 3 day and 3 Renalyte (Oral Rehydrated Solution) @ 0.5 liter(0.625gm)/adult goat orally at every 6 hours interval for 5 day.
Statistical analysis
A significant Chi-square (χ2) test was used to observe the significant influence of age, sex, breed and immune status of goat on prevalence of PPR in goat. The χ2 test was performed with the statistical software “EpiInfo”.
RESULTS AND DISCUSSION
The study was conducted among the naturally infected PPR goats of various ages and sex that were brought to the hospital and in the field. A number of 280 diseased goats were recorded of which 35 cases were PPR that were examined, treated and followed up. Various observations on different parameters were recorded. The overall prevalence of PPR in goats was 12.5% in the studied area.
Table 1
Prevalence of PPR in relation to breeds, age and immune status of goat.
| Character-istics | Prevalence (%) | ||
| Breeds | Black Bengal | 13.47 | P>0.05 |
| Jamunapari | 8.00 | ||
| Age (Months) | ≤ 6 | 6.25 | P < 0.05 |
| 7-12 | 20.87 | ||
| 13-18 | 12.72 | ||
| ≥19 | 7.142 | ||
| Immune status | Vaccinated | 1.92 | P<0.05 |
| Non- vaccinated | 14.91 |
The prevalence of PPR in relation to breed, age and immune status of goats are summarized in Table 1. The prevalence of PPR disease was higher in Black Bengal goat (13.47%) than in Jamunapari goat (8.69%). This result is in agreement with the former work of Samad (1996) who observed that, Black Bengal breed (67.24%) are more susceptible to PPR than Jamunapari (32.76%). Statistical analysis shows that the occurrence of PPR in two breeds tested is insignificant (P>0.05) indicating that the disease is uniformly distributed in both breeds.
There significant (P<0.05) variation in the occurrence of PPR in different age group of goat (Table 1). The prevalence of PPR was minimum (20.87%) in 7-12 months age group of goat followed by age groups 12.72%, 7.142% and 6.25% in ≤6 months, 13-18 months and ≥19 months age groups respectively. This result is consistent with the report of Blood, et al. (1995), who observed that the disease was rapidly fatal in the young animals (60.80%) especially in 7-12 months age group.
Significant (P<0.05) variation in the prevalence of PPR in relation to immune status of goat was observed in this study (Table 1).The prevalence of PPR was higher in non-vaccinated (14.91%) as compared with vaccinated (1.92%) goats. This result supports the earlier report made by Gibbs et al. (1979) who found higher prevalence (68.38%) of PPR in the nonvaccinated goat population. Vaccination should be given to the goat in the study area might decrease the prevalence.
The effects of PPR on lymphocyte and neutrophil count are shown in Table 2. Lymphocytes percentage was decreasing with the increase of duration of disease. Lymphocyte percentages were counted in diseased goats as 40 % and 28 % on day 3 and day 6 respectively and neutrophil percentages were as 56% and 66% on day 3 and day 6 respectively. Neurtrophil percentage gradually increased due to ratio of lymphocytes percentage decreased and also to protect body from other bacterial infection. Gradual decrease of lymphocyte count is thought to be due to destruction of lymphoid tissue because the virus has special affinity to the lymphoid tissue and destroy lymphocytes. These findings however, are in confirmatory with earlier reports of Sil et al. (1995) who observed that the lymphocytes percentage declined (up to 21%) with the onset of duration of diseases increased at the same time neutrophilia (up to 72%) occurred.
Table 2
Effects of PPR on lymphocyte and neutrophil counts.
| Sample No | Black Bengal (B); Jamunapari (J) | Lymphocyte (%)(Normal value 50-70) | Neutrophil (%)(Normal value 40-55) |
| 01 ( Day-3) | B | 40 | 56 |
| 02 ( Day-3) | J | 38 | 58 |
| 03 ( Day- 4 ) | B | 35 | 65 |
| 04 ( Day- 5) | B | 30 | 65 |
| 05 ( Day-6) | B | 28 | 66 |
Table 3
Response to different lines of treatment for PPR infected goat.
| Line of treatment/Drug used(Trade + Generic) | Response to treatment | Total case | % of response to treatment | ||
| yes | no | ||||
| Diadin (sulphadimidine-Na) + Dellergen (Promethazine HCI) + Renalyte (ORS) | 13 | 10 | 23 | 56.52 | P>0.05 |
| Renamycin- 100(Oxytetracycline) + Dellergen (Promethazine HCI) + Renalyte (ORS) | 5 | 7 | 12 | 41.66 | |
ORS (Oral Rehydrated Solution)
Figure 1
Purulent ocular and nasal discharge of PPR infected goat.
Figure 2
Purulent nasal discharge of PPR infected goat.
Figure 3
PPR infected goat in recumbancy.
Figure 4
Ulceration of buccal mucosa PPR infected goat.
Clinical signs
The clinical signs found during clinical examination of PPR patients were dullness and depression, anorexia, rough hair coat, dyspnoea, diarrhoea, soiled hind quarter, swelling and erosion of lip, occulonasal discharge and inflamed eyes. These findings coincide with the former findings observed by DEFRA (2001), Lefevre and Diallo (1990) and Ikede and Uzuokwu (1983).
Physical examination
Physical examination revealed that the PPR patients had high temperature (104°F-106°F), erosion in gum and dental pad, cheesy material on lip, ulceration of buccal mucosa, dehydration examined by skin fold test, abnormal lung sound (whistling sound) detected on indirect auscultation from lung and trachea and congested conjunctiva. These observations are in confirmatory with the earlier reports of Lefevre and Diallo (1990), Sil (2000), Rowland et al. (1969) and Brown et al. (1991).
Response to treatment
The relative effects of drugs in treatment of PPR are presented in table 3. The above results showed that, the percentage of response to treatment towards parenteral (I/M) sulphadimidine was significantly higher (56.52%) than parenteral oxytetracycline (41.66%) which is supported by the study of Gupta et al. (2007). The statistical analysis shows that the result is insignificant (P>0.05) that indicates both drugs have uniform action to cure the PPR case.
From the study, it is observed that Black Bengal goats were more susceptible to PPR than Jamunapari goats. About 7-12 months were more prone to PPR than adult. Vaccination of goat markedly reduces the chance of infection. The chances of other bacterial infections raises especially pneumonia and gastroenteritis with duration of disease increases, it may be due to immunosuppressive nature of the PPR disease. The disease potentially response to sulphadimidine and oxytetracycline. The study suggests taking preventive measures in terms of vaccination and supportive therapy with sulphanamide/ oxytetracycline in order to effective control of this disease.
REFERENCES
Alam JSMA, Rahman A and Sayeed MA (1998). A study on Livestock Credit Rural Bangladesh. Bangladesh Journal of Livestock Research, 1 (1): 15-18.
Barrett E (1994). Rinderpest and distemper viruses. In: RG Webster and A Granoff (eds) Encvci Virology, 3: 1260-1268.
Blood DC, Radostis OM and Gay CC (1995). Veterinary Medicine. 8th edition.Ballier Tindall, UK: 837.
Debnath NC (1995). Peste Des Petits Ruminants (PPR): an overview. Proceeding of the BSVER Symposium on Eradication of Rinderpest and Related Diseases. 2 December, 1995, Dhaka. PP. 9-13.
DEFRA (Department for Environment Food and Rural Affairs). Animal health and welfare peste des petits ruminants, May 24. 2005.
Gibbs EJP, Taylor WP, Lawman MJP and Bryant AP (1979). Classification of peste des petits ruminants (PPR) virus as the fourth member of the genus morbilli virus Intervirology, Veterianry Journal, 15:35-41.
Gupta SD, Biswas PK, Habib S and Debnath NC (2007). Prevalence of PPR in the greater Chittagong district of Bangladesh. Science, 3 (2): 36 – 41.
Ikede BO (1983). Histopathology of natural cases of PPR, Characteristic lesions and changes occurring during the disease peste des petits ruminants (PPR) in sheep and goats. Proceedings of the International workshop held at IITA Ibadan, Nigeria, 24-26 September. D.H Hill (eds) ILCA Addis Ababa. Ethiolpia.
Lefevre PC and Diallo A (1990). Peste des petits ruminants, Review of Science and Technology (International Office of Epizootics), 9: 951-965.
Ozkul A, Akca Y and Alkan F (2002). Prevalence, Distribution, and host range of peste des petits ruminants virus, Turke. Emergency Infectious Disease, 8(7): 708-12.
Roeder PL and Obi TU (1999). Recognizing peste des petits ruminants: a field manual. FAO Animal Health Manual, 5: 28.
Roeder PL, Abraham G, Kenfe G and Barrett T (1994). Peste des petits ruminants in Ethiopian goats. Tropical Animal Health and Production, 26 (2): 69-73.
Samad MA (1996). Poshu palon O Chikitasaviday (Animal Husbandry and Medicine).1st published by M. Bulbul, BAU Campus, Mymensingh, Bangladesh. PP. 446-449.
Sil BK, Rahman MF and Taimur MJFA (1995). Observations on outbreaks of peste des petits ruminants in organized goat farms and approach of its treatment and revention. Second BSVER Annual Scientific Conference. Programme and Acstracts. Dhaka 2-3 December, 1995. PP. 10.